
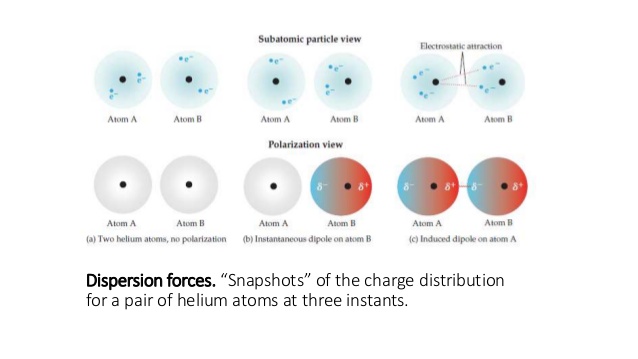

My question is: if we lower the temperature we subsequently decrease the kinetic energy of the electrons, i.e. The aforementioned statement suggests that as we lower the temperature we form a solid or a liquid meaning that the inter-molecular forces (London forces in this case)became stronger._ I learned that what results in London fores between molecules is the "cloud of electrons" that results in an instantaneous dipole and induced dipole, which eventually results into forming these weak London forces._ The molecular structure, size and number of interactions affect the strength of dispersion forces."London (dispersion) forces are responsible for the fact that non-polar substances can be condensed to form liquids and sometimes solids at low temperatures"._ The polarity differences in the bond and electronegativity differences affect the strength of dipole dipole interactions.Dispersion forces are weaker than dipole dipole interactions.Two non polar molecules can have dispersion forces and two polar molecules will have dipole dipole interactions.In contrast, dispersion forces occur in molecules where there are no permanent dipoles. Dipole dipole interactions occur between two permanent dipoles.What is the difference between Dipole Dipole Interaction and Dispersion Forces?

And this is a type of Van der Waals forces, which is separately known as London dispersion forces. This kind of interaction is known as an instantaneous dipole- induced dipole interaction. These temporary dipoles can induce a dipole in the neighboring molecule and thereafter, an interaction between opposing poles can occur. The end with the electron will have a temporarily negative charge, whereas the other end will have a positive charge. So there can be instant charge separation within the molecule if the electron moves toward one end of the molecule. However, electrons are constantly moving in these molecules. There are some symmetrical molecules like H 2, Cl 2 where there are no charge separations.
For an intermolecular attraction, there should be a charge separation. This is also known as London dispersion forces. This is known as dipole dipole interaction. When the positive end of one molecule and the negative end of another molecule are close by, an electrostatic interaction will form between the two molecules. The atom with a higher electronegativity gets the slight negative charge, and the atom with a lower electronegativity will get the slight positive charge. At this instance, we say that the atoms have obtained a partial negative or positive charge (dipole). Because of the uneven sharing of electrons, one atom will have a slightly negative charge whereas the other atom will have a slightly positive charge. This will result in an unequal distribution of electrons between the two atoms. Therefore, the bond electron pair is pulled more by one atom compared to the other atom, which is participating in making the bond. When the two atoms that form a bond are different, their electronegativities are often different. Down the group, the electronegativity values decrease. Therefore, halogens have larger electronegativity values in a period, and group 1 elements have comparatively low electronegativity values. From left to right through a period, the electronegativity value increases. Fluorine has the highest electronegativity value, which is 4 according to the Pauling scale. In the periodic table, there is a pattern as to how the electronegativity values are changing. Usually Pauling scale is used to indicate the electronegativity values. Electronegativity gives a measurement of an atom to attract electrons in a bond. Polarity arises due to the differences in electronegativity. These bonds determine the behavior of molecules. However, all of these intermolecular interactions are weaker than the intramolecular forces like covalent or ionic bonds. Some intermolecular forces are strong while some are weak. Dipole Dipole vs Dispersion | Dipole Dipole Interactions vs Dispersion Forcesĭipole dipole interactions and dispersion forces are intermolecular attractions between molecules.


 0 kommentar(er)
0 kommentar(er)
